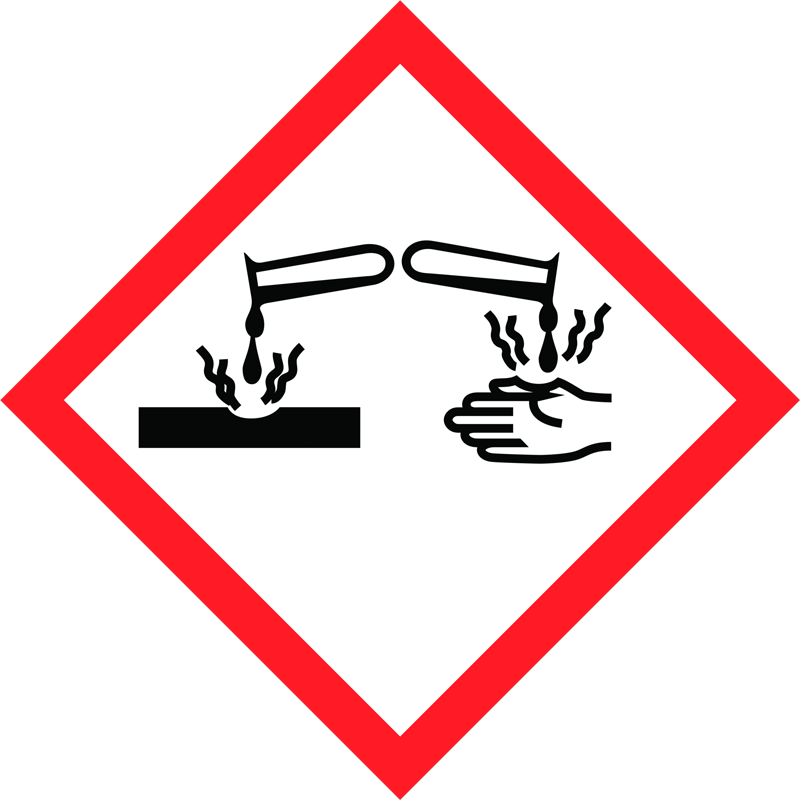
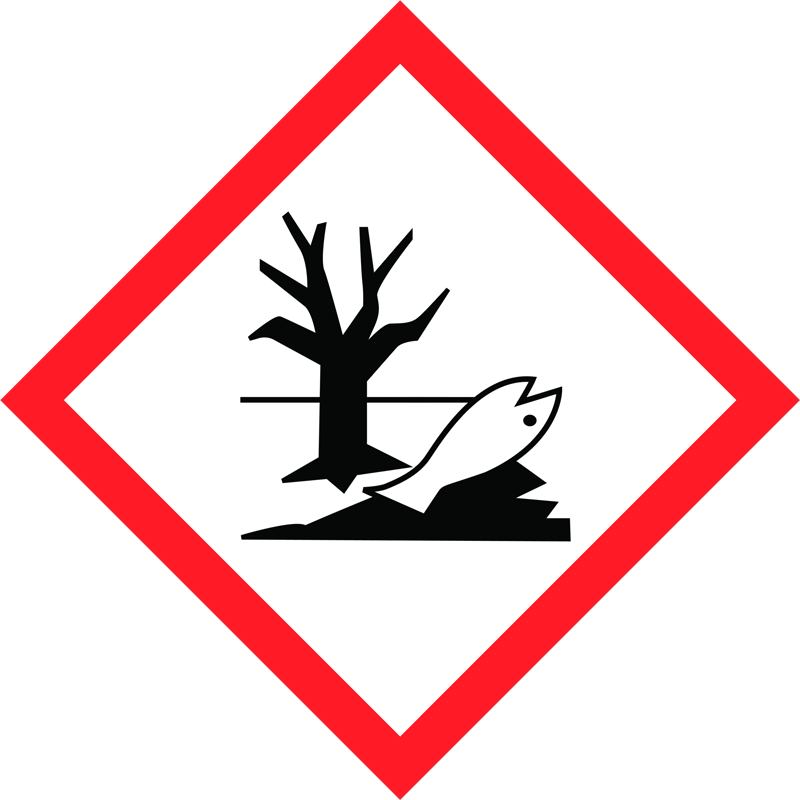
 Chlorine is a very reactive element. It has been classified as so toxic that it is in the highest toxicity category. It also bleaches out dyes. (find out more here (German))
Chlorine is a very reactive element. It has been classified as so toxic that it is in the highest toxicity category. It also bleaches out dyes. (find out more here (German))
Drinking water in Switzerland is allowed to have a maximum chlorine content of 0.1 mg/litre.
Pool water needs a concentration of 0.5-1.2 mg/litre chlorine, depending on the temperature, meaning that pool water treated with chlorine must be disposed of via the sewage system, because the water exceeds the limit values by 5-12 times and is highly harmful to the environment.
Chlorine stimulates the build-up of limescale in the pipe systems and the pump, which greatly reduces the life expectancy of the latter.
In other words: chlorine kills organisms, which is why the pool water remains clear.
If the pH of the water rises above 8, the effect of chlorine is severely limited, even at high doses.
This is why the second chemical comes into play: ph-minus (e.g. sodium bisulphate or sulphuric acid).
What does chlorine do to the human organism? Red eyes, dry skin, bleached hair and skin are all symptoms of caustic reactions. Children also often swallow pool water when playing: what does this do to our bodies?
If you are interested, I refer you to the German Green-Peace Studie.
Salt electrolysis produces sodium hydroxide, chlorine and hydrogen from common salt and water. It is safer and easier to use - the result is about the same.




